2024. 9. 20. 18:30ㆍScience/Biology
https://www.nature.com/articles/nature12517 (28 August 2013)
Cerebral organoids model human brain development and microcephaly
Abstract
The complexity of the human brain has made it difficult to study many brain disorders in model organisms, highlighting the need for an in vitro (실험 환경 상에서) model of human brain development. Here we have developed a human pluripotent stem cell-derived (줄기세포) three-dimensional organoid culture system, termed cerebral organoids, that develop various discrete, although interdependent, brain regions.
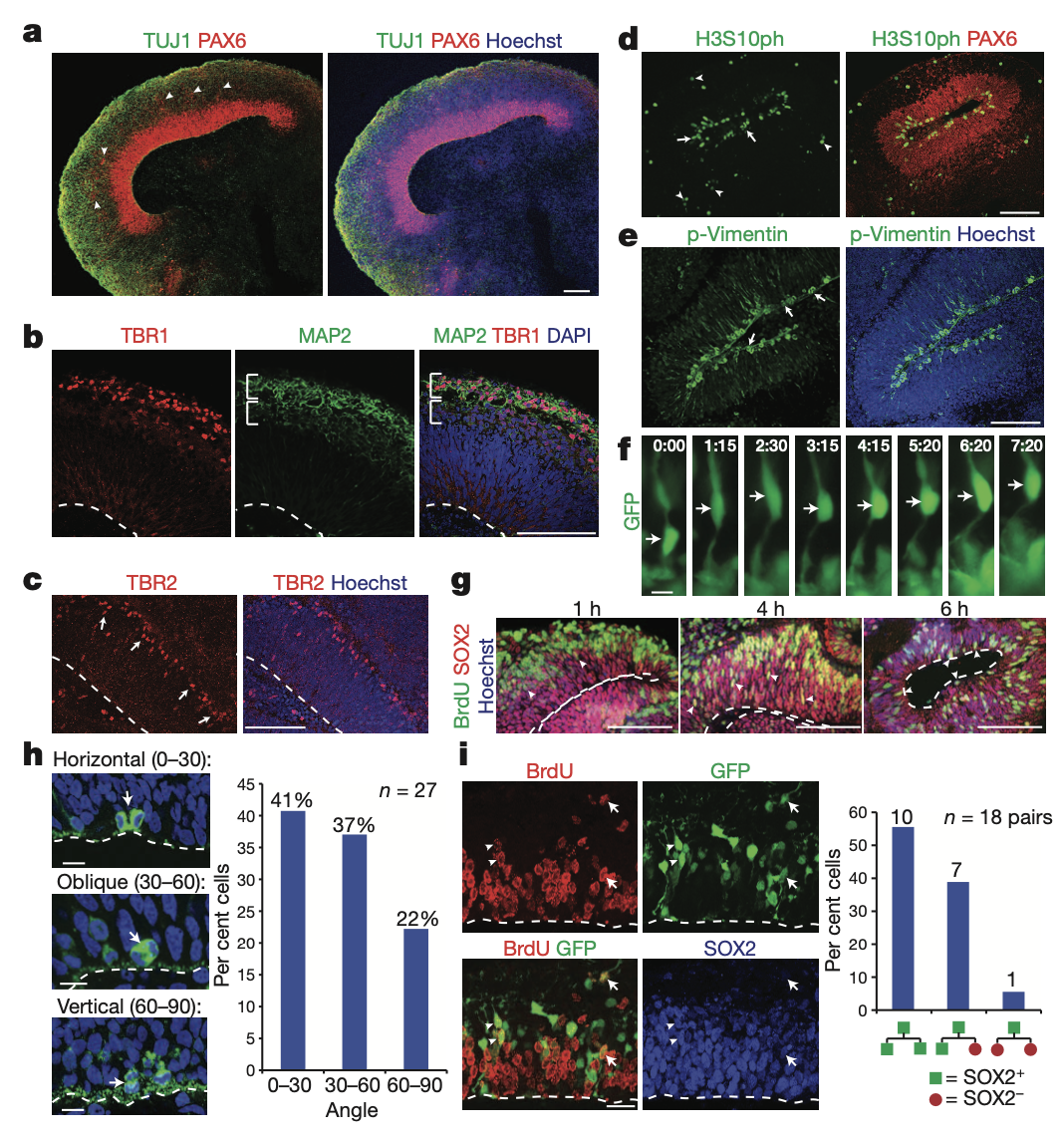
These include a cerebral cortex (대뇌피질) containing progenitor (전구세포) populations that organize and produce mature cortical neuron subtypes. Furthermore, cerebral organoids are shown to recapitulate features of human cortical development, namely characteristic progenitor zone organization with abundant outer radial glial stem cells(방사형 신경 아교 줄기세포, RGPs).
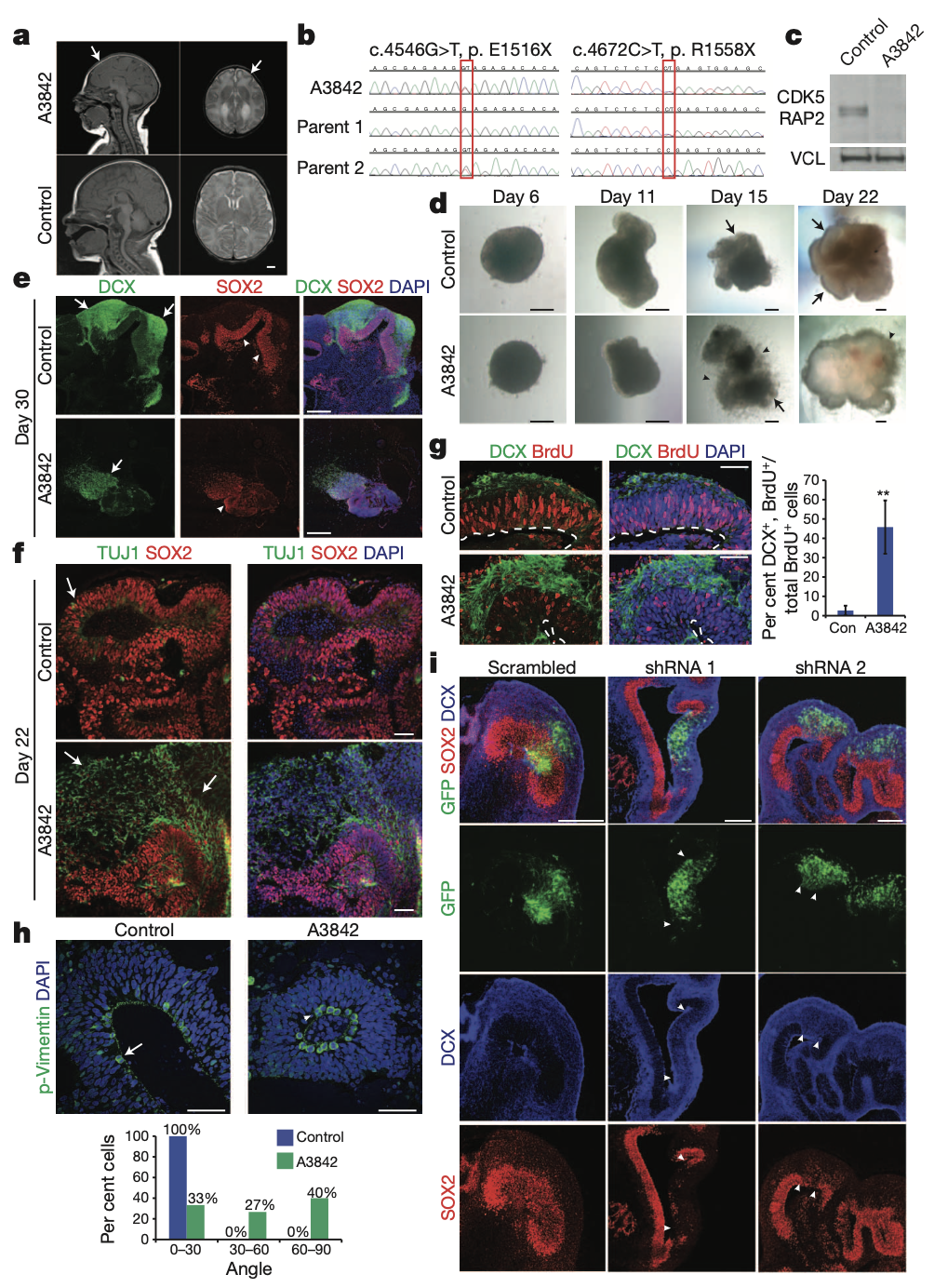
Finally, we use RNA interference and patient-specific induced pluripotent stem cells to model microcephaly (소두증), a disorder that has been difficult to recapitulate in mice. We demonstrate premature neuronal differentiation in patient organoids, a defect that could help to explain the disease phenotype (표현형). Together, these data show that three-dimensional organoids can recapitulate development and disease even in this most complex human tissue.
* Radial Glial Stem Cells, RGPs : 방사형 아교 세포
- 대뇌 피질의 모든 뉴런을 생성하는 역할을 하는 전구 세포
- bipolar-shaped progenitor cells that are responsible for producing all of the neurons in the cerebral cortex.
* RNA interference : RNA 간섭
- 세포 내에서 활성화 상태의 유전자와 그렇지 않은 유전자를 구별하는 역할
Discussion
We have established a novel approach to studying human neurodevelopmental processes through in vitro culture of cerebral organoids from human pluripotent stem cells (만능 줄기세포, PSCs). This method recapitulates not only fundamental mechanisms of mammalian (포유류의) neurodevelopment, but also displays characteristics of human brain development. We are hopeful that this method will allow for the study of a variety of neurodevelopmental processes specific to human brain development.
Furthermore, a primary goal in neuroscience is to understand the roots of human neurological disease. We have modelled at least some aspects of microcephaly in these cerebral organoids. The finding that progenitor zones (신경전구세포) in patient-derived tissues display premature neural differentiation at the expense of early progenitors supports a model in which the founder population of RG progenitors fails to properly expand in patient tissues, thereby leading to an overall smaller brain. -> 소두증의 발현
This may also explain why mouse models have been unable to recapitulate the severity of the disorder in humans. It is proposed that the mouse founder population of neural progenitors do not undergo expansion to the same extent as in humans before the onset of neurogenesis7. Thus, a disruption of the founder population in mice would not lead to as severe an effect as that seen in humans.
Overall, our findings suggest that we can use this in vitro culture system to model aspects of human neurodevelopment and neurological disease and hopefully provide novel insight into the pathogenesis of these disorders.
* progenitor zones - 기능에 대해 알아보기
* RG progenitors -> 인간과 쥐의 차이점에 대해 더 알아보기 (소두증 발현 과정에서)
Introduction
Mammalian brain development begins with the expansion of the neuroepithelium (신경상피) to generate radial glial stem cells (RGs - 방사형 신경아교 줄기세포)1. These RGs divide at the apical (정점의) surface within the ventricular zone (VZ, 심실대) to generate neurons and intermediate progenitors (전구세포). Intermediate progenitors populate the adjacent subventricular zone (SVZ, 인접한 뇌실하대), whereas neurons migrate through the intermediate zone to populate specific layers within the cortical plate (피질판).
* progenitor cell : 전구세포 -> 잘 이해 X
- 포유류의 세포는 체세포, 생식세포, 줄기세포 3가지 (성인은 약 100조개의 세포로 구성)
- 체세포 - 염색체 쌍을 가지는 이배체 - 피부와 근육 등 대부분의 인체 구성 / 생식세포 - germ line cell을 따라 생겨남
- 줄기세포 - 특화된 어떠한 세포로도 분화 가능
- 만능줄기세포는 다능한(multipotent) 전구체 세포(progenitor cell)로 분화
- 전구체 세포는 실제 조직에서 기능하는 세포로 분화
ex) 골수의 조혈모 줄기세포는 성체 줄기세포로, 적혈구, 백혈구, 혈소판으로 분화
- 골수의 중간엽 줄기세포는 성체 줄기세포로 기질 세포, 지방 세포, 뼈 세포가 됨
- 상피 줄기세포는 전구체 세포로 피부 세포들로 분화 / 근육 위성 세포는 전구체 세포로 근육 조직으로 분화
In humans, the organization of progenitor zones is markedly more elaborate; the SVZ is split by an inner fibre layer (IFL) into an inner SVZ and an outer SVZ (OSVZ)2. The OSVZ represents an entirely separate progenitor layer populated by intermediate progenitors and a unique stem cell subset termed outer radial glia (oRG)3,4, which are only present to a limited degree in rodents5. Both the IFL and OSVZ are completely absent in mice6. These key differences allow for the striking expansion in neuronal output and brain size seen in humans7,8.
Primary microcephaly is a neurodevelopmental disorder in which brain size is markedly reduced9. (소두증의 정의) Autosomal-recessive mutations (상염색체 열성 돌연변이) have been identified in several genes, all of which encode proteins localizing to the mitotic spindle apparatus (체세포 분열 방추체). Heretofore 지금까지, primary microcephaly pathogenesis (병인, 질병이 발생하는 과정) has primarily been examined in mouse models. However, mouse mutants for several of the known genes11–14 have failed to recapitulate the severely reduced brain size seen in human patients. (쥐로는 인간의 소두증을 설명할 수 X, 차이가 크다.)
Given the dramatic differences between mice and humans, methods that recapitulate paradigms of human brain development in vitro have enormous potential. Although considerable progress has been made for in vitro models of whole-organ development for other systems, a three-dimensional culture model of the developing brain as a whole has not been established. Previous studies have modelled certain isolated neural tissues in vitro19–23, including dorsal cerebral cortical tissue, which could recapitulate many aspects of early development.
However, later events such as the formation of discrete cortical layers with stereotypical inside-out organization as well as human characteristics, such as the presence of oRG cells and the unique organization of progenitor zones, were not described. Here we describe a three-dimensional culture system for deriving brain tissue in vitro. These so-called cerebral organoids develop a variety of regional identities organized as discrete domains capable of influencing one another. Furthermore, cerebral cortical regions display an organization similar to the developing human brain at early stages, as well as the presence of a considerable oRG (outer radial glia) population. Moreover, cerebral cortical neurons mature to form various pyramidal identities with modest spatial separation. Finally, we use patient-derived induced pluripotent stem (iPS) cells and short hairpin RNA (shRNA) in these organoids to model CDK5RAP2-dependent pathogenesis of microcephaly, a disorder that has been difficult to model in mice.
Generation of cerebral organoids
Recent progress with in vitro models of various organ systems has demonstrated the enormous self-organizing capacity for pluripotent stem cells to form whole tissues. We built upon this concept and developed a protocol without the use of patterning growth factors, focusing instead on improving growth conditions and providing the environment necessary for intrinsic cues to influence development.
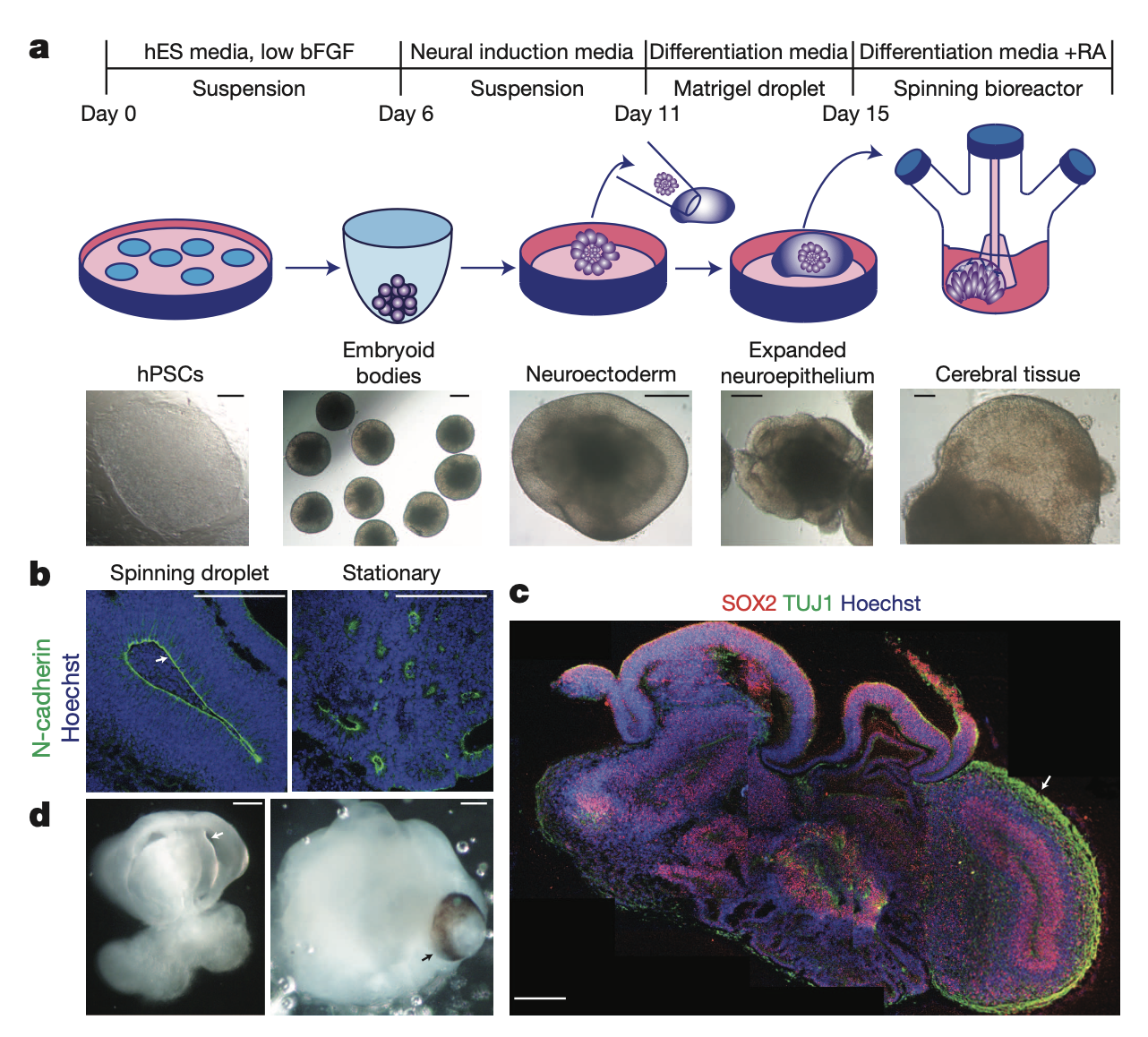
We began with a modified approach to generate neuroectoderm (외배엽) from embryoid bodies(배아체). Neuroectodermal tissues were then maintained in three-dimensional culture and embedded in droplets of Matrigel to provide a scaffold for more complex tissue growth. These Matrigel droplets were then transferred to a spinning bioreactor to enhance nutrient absorption (Fig. 1a). This method led to rapid development of brain tissues, which we termed cerebral organoids, requiring only 8–10 days for the appearance of neural identity and 20–30 days for defined brain regions to form. -> 빠르게 오가노이드를 성장시킬 수 있음
Cerebral organoids at early stages (15–20 days) formed large, continuous neuroepithelia(신경아교세포) surrounding a fluid-filled cavity reminiscent of a ventricle (뇌실) with characteristic apical localization of the neural specific N-cadherin (Fig. 1b). Furthermore, the neuroepithelium was larger and more continuous than tissues generated using a stationary approach, which instead formed an aggregate of several small rosette-like neuroepithelia.
Although tissues reached maximal size by 2 months, cerebral organoids formed large (up to 4 mm in diameter), complex heterogeneous tissues, which could survive indefinitely (currently up to 10 months) when maintained in a spinning bioreactor. Histological and gross morphological analysis revealed regions reminiscent of cerebral cortex, choroid plexus(맥락 얼기, 뇌척수액 생성), retina and meninges(뇌척수막) (Fig. 1c, d and Extended Data Fig. 1b).
Notably, tissues typically reached a size limit, probably because of the lack of a circulatory system and limitations in oxygen and nutrient exchange. Consistent with this, extensive cell death was visible in the core of these tissues (Extended Data Fig. 1c), whereas the various brain regions developed along the exterior. Furthermore, cerebral organoids could be reproducibly generated with similar overall morphology and complexity from both human embryonic stem (ES) cells and iPS cells (Extended Data Fig. 1d, e).