2024. 9. 24. 21:28ㆍScience/Biology
Cardioids reveal self-organizing principles of human cardiogenesis
Cardioids that pattern and morph into chamber-like structures are established from human pluripotent stem cells. (Hofbauer et al., 2021, Cell)
https://www.cell.com/cell/fulltext/S0092-8674(21)00537-7

* Self-organizing이 중요한 이유 -> 단순히 세포가 막 증식하는 과정 X
-> organ 구조의 형태가 잡혀가면서 오가노이드로 성장 -> self-patterning + self-morphogenesis
- 해당 과정 (cardioids에서는 cavity, 심장 혈관과 좌심실에 focus)을 밝히는 것이 중요한 이유
Abstraction
Organoids capable of forming tissue-like structures have transformed our ability to model human development and disease. With the notable exception of the human heart, lineage-specific self-organizing organoids have been reported for all major organs.
Here, we established self-organizing cardioids from human pluripotent stem cells that intrinsically specify, pattern, and morph into chamber-like structures containing a cavity. Cardioid complexity can be controlled by signaling that instructs the separation of cardiomyocyte (심근세포) and endothelial layers (내피) and by directing epicardial (심외막의) spreading, inward migration, and differentiation.
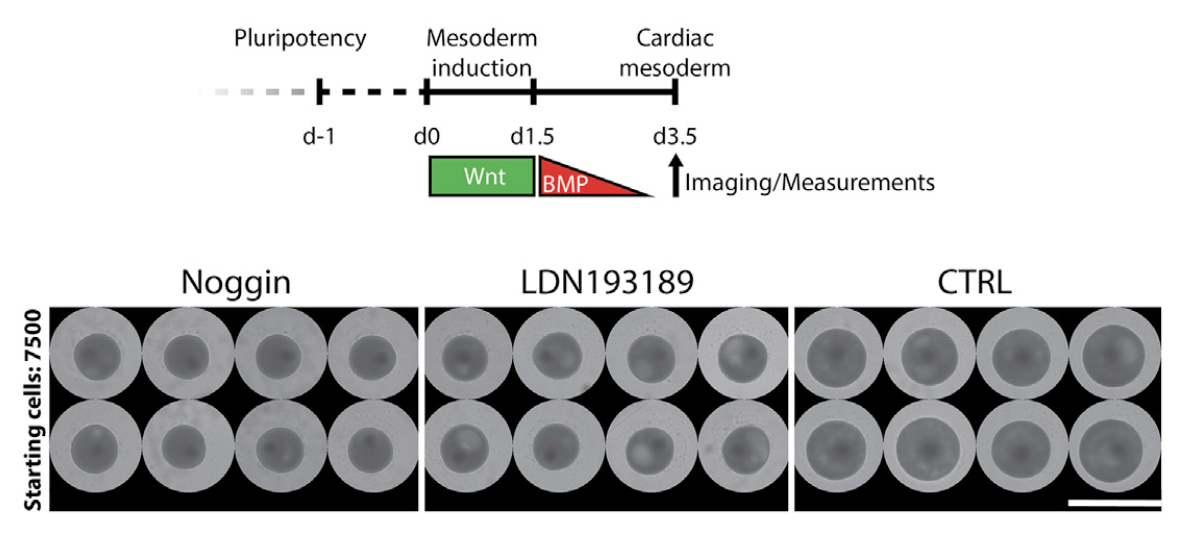
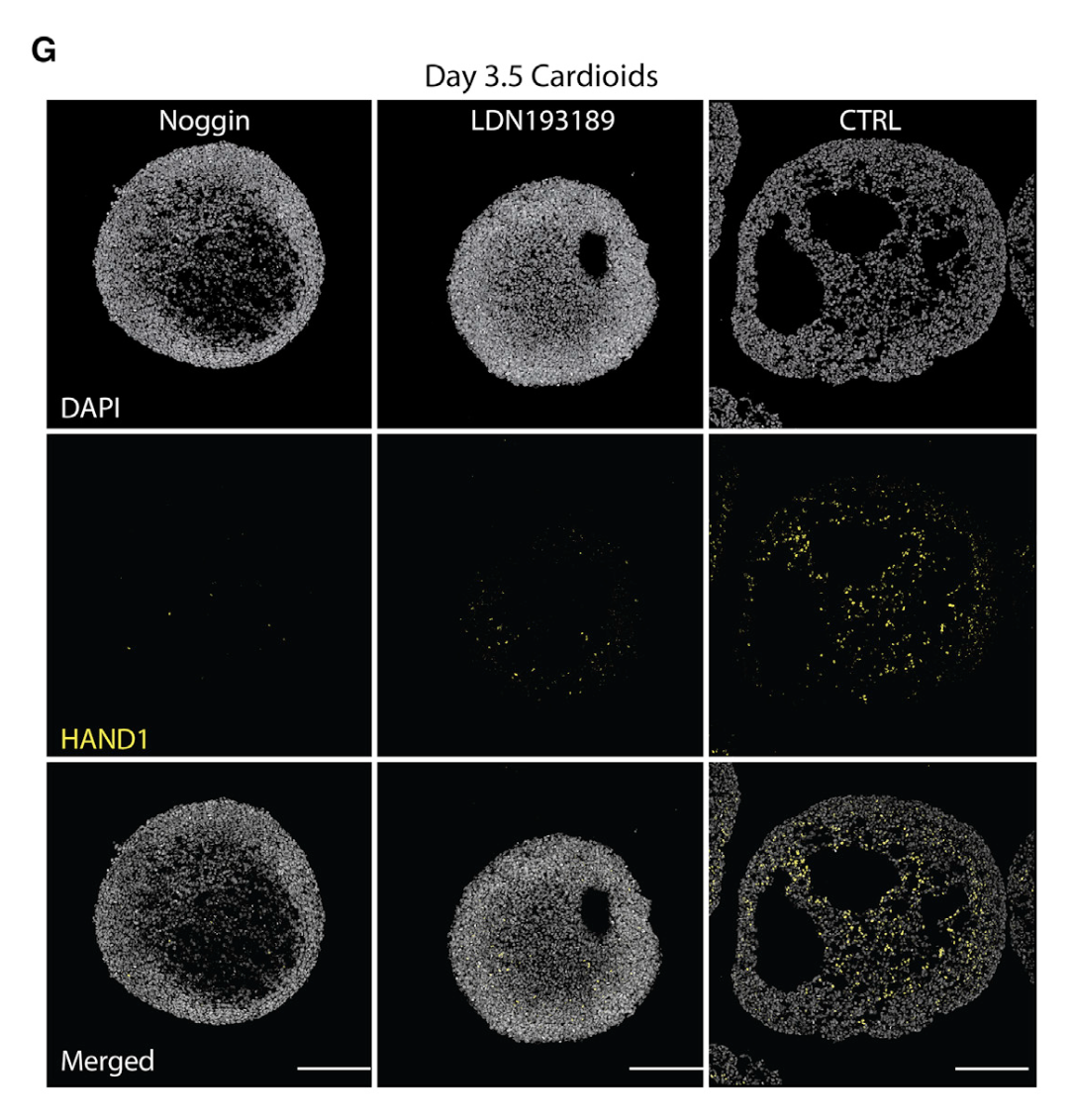
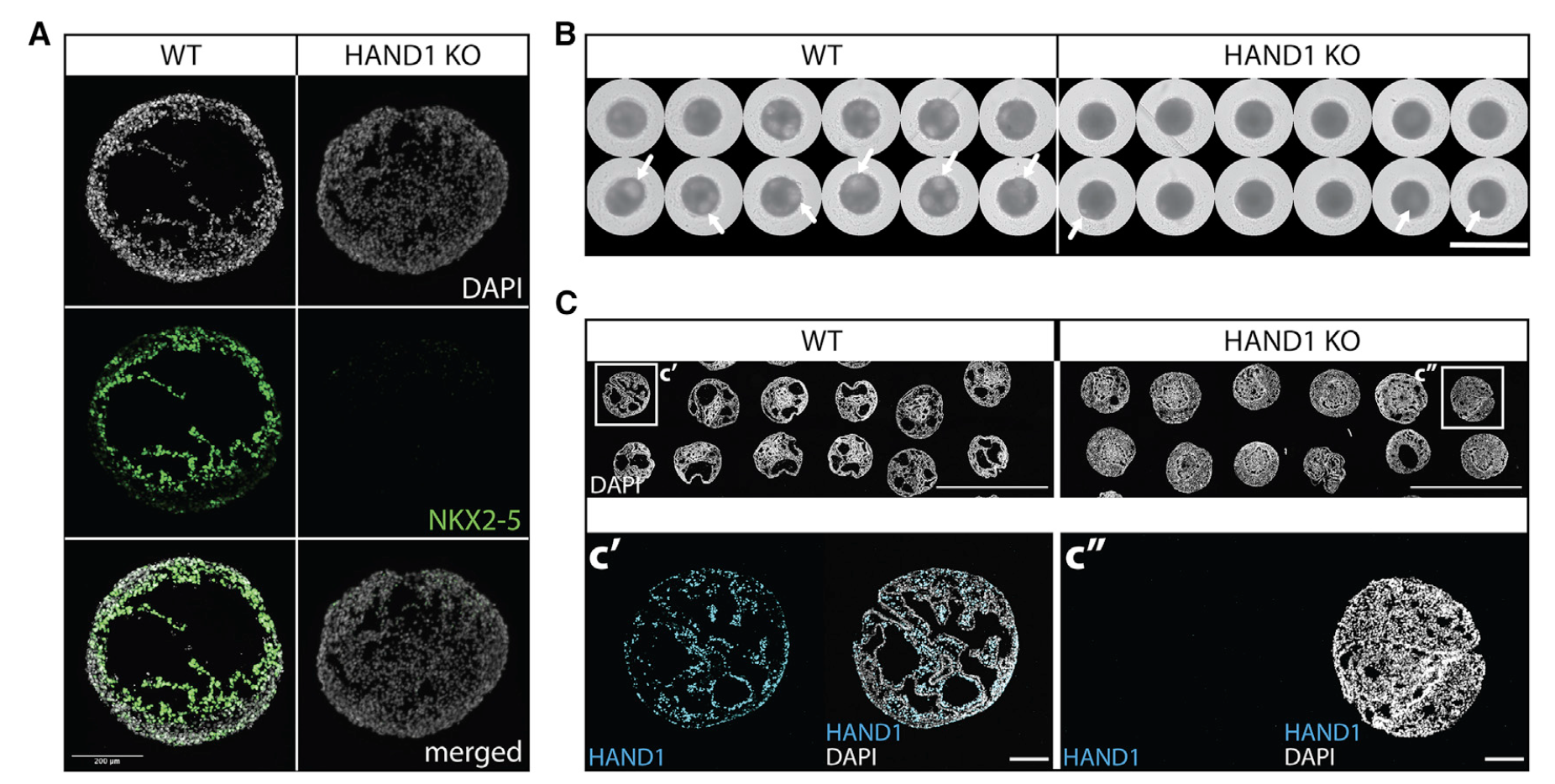
We find that cavity morphogenesis is governed by a mesodermal WNT-BMP signaling axis and requires its target HAND1, a transcription factor linked to developmental heart chamber defects. (심실 구조의 발달과 연관성을 밝힘)
Upon cryoinjury (냉동 손상), cardioids initiated a cell type-dependent accumulation of extracellular matrix(세포외기질), an early hallmark of both regeneration and heart disease. Thus, human cardioids represent a powerful platform to mechanistically dissect(해부하다) self-organization, congenital heart defects (선천성 심장 결함) and serve as a foundation for future translational research.
Introduction
The human heart, the first functional organ to form in development, is one of the most difficult organs to model in vitro (Lancaster and Huch, 2019; Schutgens and Clevers, 2020). Malformations of the heart are by far the most common human birth defects (Majumdar et al., 2021) but their developmental etiology is poorly understood (Nees and Chung, 2020). These often-dramatic morphogenetic disorders can be caused by mutations that affect the activity of cardiogenic signaling pathways and transcription factors during early embryogenesis (Kelly et al., 2014; Meilhac and Buckingham, 2018; Zaidi and Brueckner, 2017).
From work in animal and cellular models, we know how the cardiac lineage (심장 계통) is specified in a stage-specific manner from embryonic mesoderm(배아 중배엽) to produce cardiomyocytes (CMs, 심근세포), endocardial cells (ECs, 심내막세포), and epicardial cells (심외막세포). However, how signaling directs these cell types to self-organize into layers and shape a heart chamber (심실), or fail in cardiac defects, remains unclear.
-> 해당 연구가 밝혀낼 부분
These questions are challenging to tackle in complex systems, because manipulating signaling pathways at the spatial and temporal resolution required to dissect rapid and complex developmental processes is not yet feasible. Thus, we need in vitro models that mimic aspects of development but are simple enough to resolve the intricate dynamics of cardiogenesis and its malformations in humans.
Human organoids have been used to dissect (해부/분석하다) mechanisms of patterning and morphogenesis in multiple tissues and organs. These structures emerge in culture from human pluripotent stem cells (hPSCs) or adult stem cells and are coaxed by (영향을 받은) signaling to form a tissue-like architecture, organ-specific cell types, and exert organ-specific functions (Clevers, 2016; Lancaster and Knoblich, 2014) for use in numerous applications (Lancaster and Huch, 2019; Schutgens and Clevers, 2020).
Their hallmark feature is the capability of self-organization that is driven by intrinsically coordinated specification, patterning, and morphogenesis (Sasai, 2013), in the absence of spatial constraints and interactions with other embryonic tissues. These properties allow the dissection of complex developmental processes in an organ-specific context as embryonic redundancies (여분) and compensation mechanisms are removed.
// organoids 관련 선행 연구들
For instance, pioneering work using optic cup organoids elucidated the morphogenesis of the eye (Eiraku et al., 2011; Nakano et al., 2012), gut organoids have been used to tease apart the initiation of intestinal crypt morphogenesis (Serra et al., 2019), and cerebral organoids allow the study of the etiology of human brain malformations (Lancaster et al., 2013). -> 읽었던 논문! :)
By harnessing developmental mechanisms, self-organization allows not only the study of organogenesis and its defects but results in more physiological models for a much wider range of diseases and applications (Lancaster and Huch, 2019; Tuveson and Clevers, 2019). -> 오가노이드로 밝혀내는 형태 형성적 측면
Although self-organizing organoids have been reported for almost all major organs, there are currently no cardiac-specific self-organizing human cardiac organoids that autonomously pattern and morph into an in vivo-like structure (Lancaster and Huch, 2019; Schutgens and Clevers, 2020).
Bioengineering approaches have successfully been applied to create artificially engineered heart tissues (often termed heart/ cardiac organoids, microchambers, etc.) using scaffolds, molds, geometric confinement, and protein matrices (Ma et al., 2015; Mills et al., 2017; Ronaldson-Bouchard et al., 2018; Tiburcy et al., 2017; Zhao et al., 2019). These have proven immensely useful to measure contraction force, perform compound screens, and model structural muscle and arrhythmogenic (부정맥) disorders.
Similarly, mouse and human PSC-derived 3D cardiac models including spherical aggregates (microtissues) of CMs and other (cardiac) cell types (Giacomelli et al., 2017, 2020; Richards et al., 2020) have been established as promising high throughput tools for drug discovery.
Moreover, recent embryoid models (Drakhlis et al., 2021; Rossi et al., 2021; Silva et al., 2020) rely on complex self-organization of non-cardiac (e.g., foregut endoderm) and cardiac lineages and thus provide further insights into germ layer interactions during early organogenesis. // 기존의 접근 방식들 about 심장계통 연구
However, existing models do not recapitulate cardiac-specific self-organization to acquire in vivo-like architecture such as a CM chamber (심근방) with inner endocardial cavity lining and are therefore limited as models of human cardiogenesis and heart disease.
* 해당 연구의 의의
Here, we established hPSC-derived self-organizing ‘‘cardioids’’ <- 연구 대상 that recapitulate chamber-like morphogenesis in the absence of non-cardiac tissues and use them to study mechanisms of human cardiogenesis and heart disease.


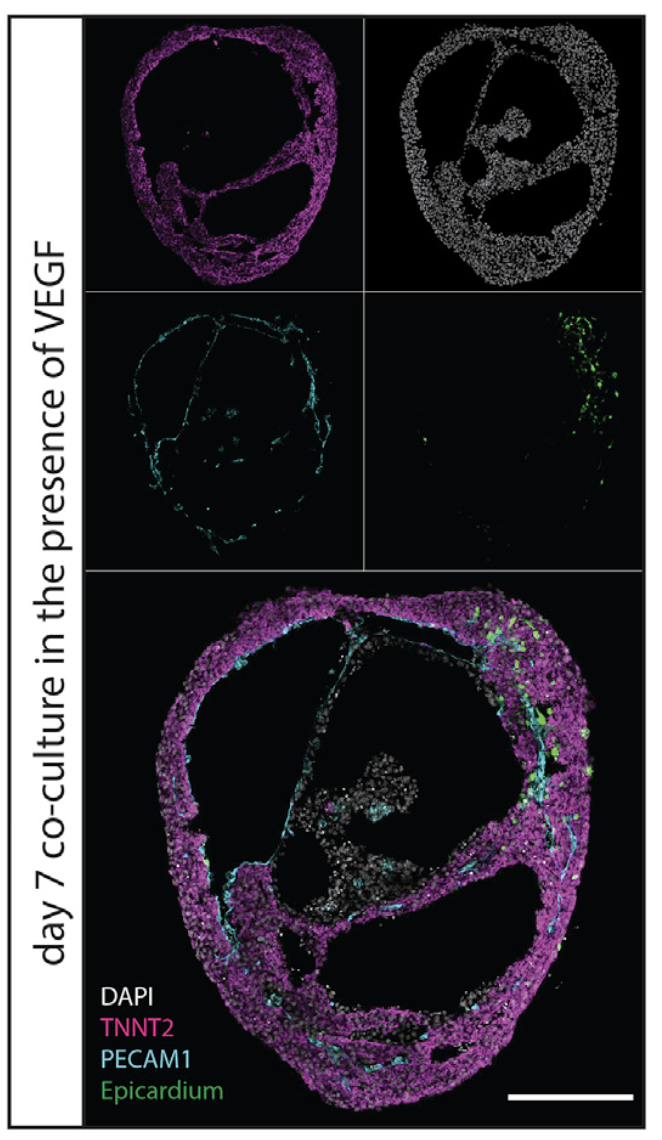
Discussion
The variability and complexity of self-organizing organoid sys- tems still hinders quantitative modeling of morphogenetic de- fects (Little and Combes, 2019). In cardioids, we address this challenge by omitting exogenous ECM and using a high- throughput approach to reach optimal signaling conditions. We further increased reproducibility by tightly controlling self-orga- nization via signaling and the stepwise incorporation of the three main cardiac lineages into cardioids. This approach allows dissecting, with high statistical power, when and where the func- tions of specific factors are required. The simplicity and modu- larity of the system that can contain either one, two, or three cardiac lineages, without interference of non-cardiac tissues as in more complex embryoid models (Drakhlis et al., 2021; Rossi et al., 2021), makes it possible to reduce self-organization and its underlying molecular and cell biological mechanisms to its bare essentials. Complexity in cardioids can therefore be tailored to the biological question asked. This is an important advantage for an organoid model, because complex biological systems often employ redundant mechanisms that are otherwise chal- lenging to tease apart.
Cardioids, as all other self-organizing organoid systems, reca- pitulate some aspects of development but also differ from embryogenesis in others. Self-organization encompasses only a subset of intrinsic developmental mechanisms, which are suffi- cient to recapitulate aspects of the in vivo-like architecture (Sasai, 2013). Consequently, using cardioids, we showed that car- diac mesoderm alone, instructed by signaling, is sufficient to form a chamber-like cavity in vitro. We propose that this cavity could be analogous to the cavity of the heart tube and early left ventricular heart chamber. In vivo, the first cavity arises from foregut endo- derm-assisted migration and fusion of bilateral cardiac mesoderm and endocardial tubes into a single heart tube (Abu-Issa and Kirby, 2007). However, bilateral heart tubes and chambers can form in the absence of either endocardium (Ferdous et al., 2009) or foregut endoderm constriction (DeHaan, 1959; Li et al., 2004), but the mechanism is still unknown. This indicates the inherent capability of cardiac mesoderm to intrinsically form cavities and chambers in vivo (Ivanovitch et al., 2017), which is in agreement with the self-organization we observed in cardioids and chick em- bryo explants in vitro. Lateral plate mesoderm, a subtype comprising cardiac mesoderm, has a similar potential to form a cavity—the pericardial body cavity (Schlueter and Mikawa, 2018). Thus, cavitation is likely a more general characteristic of mesoderm that could be called on in embryos with a foregut defect. Finally, the HAND1 KO cavity formation phenotype dem- onstrates the heart chamber defect modeling potential of cardi- oids (Grossfeld et al., 2019; McFadden et al., 2005; Risebro et al., 2006).
We used the cardioid platform to demonstrate that WNT and BMP drive chamber-like self-organization. These pathways are known to regulate cardiac specification in vivo and in vitro(Meilhac and Buckingham, 2018) but whether and at what stage they control cardiac patterning and morphogenesis was unclear. The surprising finding that early mesodermal WNT controls later cardiac self-organization is consistent with early cardiac line- age diversification during mesoderm induction in vivo (Garcia- Martinez and Schoenwolf, 1993; Ivanovitch et al., 2021; Lescroart et al., 2018). Patterning and morphogenesis occur in parallel with specification but they are not necessarily linked. In agreement with this notion, cavities can self-organize in the absence of car- diac specification and in HAND1 KO cardioids there is a defect in self-organization but not in CM specification. Conversely, inhi- bition of WNT signaling at the cardiac mesoderm stage is essential for CM specification but does not regulate cardioid self-organiza- tion. Cardioids are therefore a powerful system to intrinsically dissect regulation of specification and morphogenesis. At the same time, cardioids are simple enough to determine sufficiency of a factor for one of these processes and are thus complementary to more complex systems.
We found that WNT, ACTIVIN, and VEGF control CM and EC self-organization in cardioids. In vivo, cardiac ECs first form endocardial tubes, later become separated from the outer CM tube by an ECM-filled (cardiac jelly) interspace, and finally form the inner lining of the heart chambers (Abu-Issa and Kirby, 2007; Ivanovitch et al., 2017). How signaling coordinates these patterns and morphogenetic processes with specification was unclear. In cardioids, the patterning and morphogenesis of CM and EC lineages is controlled by the dosage of WNT and ACTI- VIN at the earliest stage of mesodermal differentiation and by VEGF that directs both specification and patterning of the EC layer in cardiac mesoderm. Interestingly, the same range of lower WNT and ACTIVIN signaling dosages stimulated ventricu- lar specification and EC lining formation suggesting a potential coordination between these processes. Inner lining of a cardiac chamber-like structure has not been observed before in neither cardiac microtissues (Giacomelli et al., 2017) nor more complex foregut-heart organoids and gastruloids (Drakhlis et al., 2021;
Rossi et al., 2021). Generation of a separate EC layer/lining in the context of a chamber cavity is crucial for activation of mecha- nosensing in vivo. Cardiac chamber mechanobiology is required for physiological EC and CM crosstalk, driving the next stages of heart development including trabeculation, myocardial compac- tion, and interaction with epicardium (Wilsbacher and McNally, 2016). Cardioids are therefore a promising system to study the underlying mechanisms of CM and EC patterning and crosstalk in the context of a beating chamber.
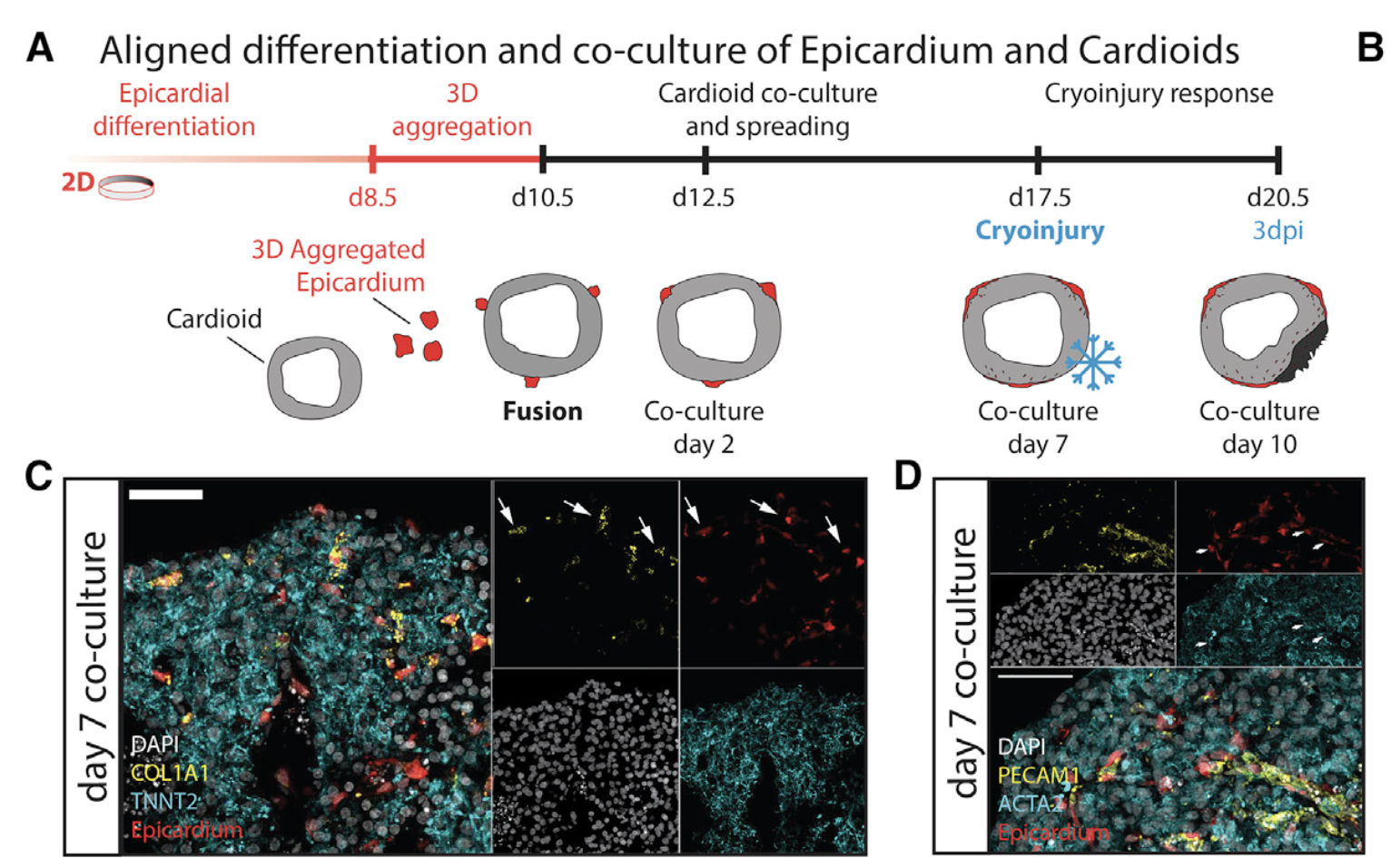
By co-culturing cardioids with epicardium, we observed epicardial spreading, migration, and differentiation reminiscent of these processes in vivo (Cao and Poss, 2018; Simo ̃ es and Riley, 2018). Epicardial and CM co-cultures have been studied before using microtissues (Guadix et al., 2017) but not in the context of a cardiac chamber-like model. This aspect is impor- tant because the crosstalk between derivatives of the epicardial, EC, and CM lineages is dependent on the mechanobiology of the heart chamber (Wilsbacher and McNally, 2016). We therefore propose that self-organization of the three cardiac lineages in chamber-like cardioids will be important to reignite the develop- mental and regenerative crosstalk that drives growth, matura- tion, and pathophysiological responses of the heart as in vivo. The striking difference in response on cryoinjury between bio- engineered organoids (Voges et al., 2017) and self-organizing cardioids supports the argument that developmental mecha- nisms impact later pathophysiology.
Limitations of study
To build the cardioid platform as a resource, we focused primarily on the FHF lineage and the earliest stages of cardiogenesis, in line with the chronology of embryonic development. However, as heart development progresses, additional lineages (second heart field, cardiac neural crest), cell types (pacemakers, immune cells), and structures (atria, right ventricle, outflow/inflow tracts) become integrated into the heart, and most congenital diseases arise during these later stages (Kelly et al., 2014; Meilhac and Buckingham, 2018). Certain aspects of cardiogenesis also depend on interactions with other germ layers, which can be spe- cifically studied with other models (Drakhlis et al., 2021; Rossi et al., 2021; Silva et al., 2020). Cardioids therefore provide a foun- dation for incorporation of additional cardiac lineages and struc- tures by signaling controlled self-organization but in the present form, are unlikely yet to faithfully recapitulate most cardiac de- fects. However, we envision that increased complexity using developmental principles will facilitate further in vitro maturation and functionalization that could render cardioids transformative for cardiovascular research. In this context, the human cardioid injury model still does not correspond to regenerative fetal and neonatal in vivo injury models and will therefore require further development and maturation. More broadly, cardioids are com- plementary to existing cardiac models as well as embryoids and comprise a significant advance in cardiac-specific organoid technology akin to what has been achieved for other organs.
Overall, the cardioid platform has a wide potential to explore fundamental mechanisms of self-organization and congenital defects as well as to generate future mature and complex human heart models suitable for drug discovery and regenerative medicine.